
Gases as we know have a good amount of space between each particle, they have very weak bonds or no bonds at all. This process of a liquid changing to a gas is called evaporation. Liquids still have a definite volume and is difficult to compress.Ī liquid can be converted to gas through heating at constant pressure to the substance’s boiling point or through the reduction of pressure at a constant temperature. In liquids, the particles are loosely packed when compared to solids which give liquids an ability to flow and take the shape of its container. A solid can also change directly into a gas through a process called sublimation.
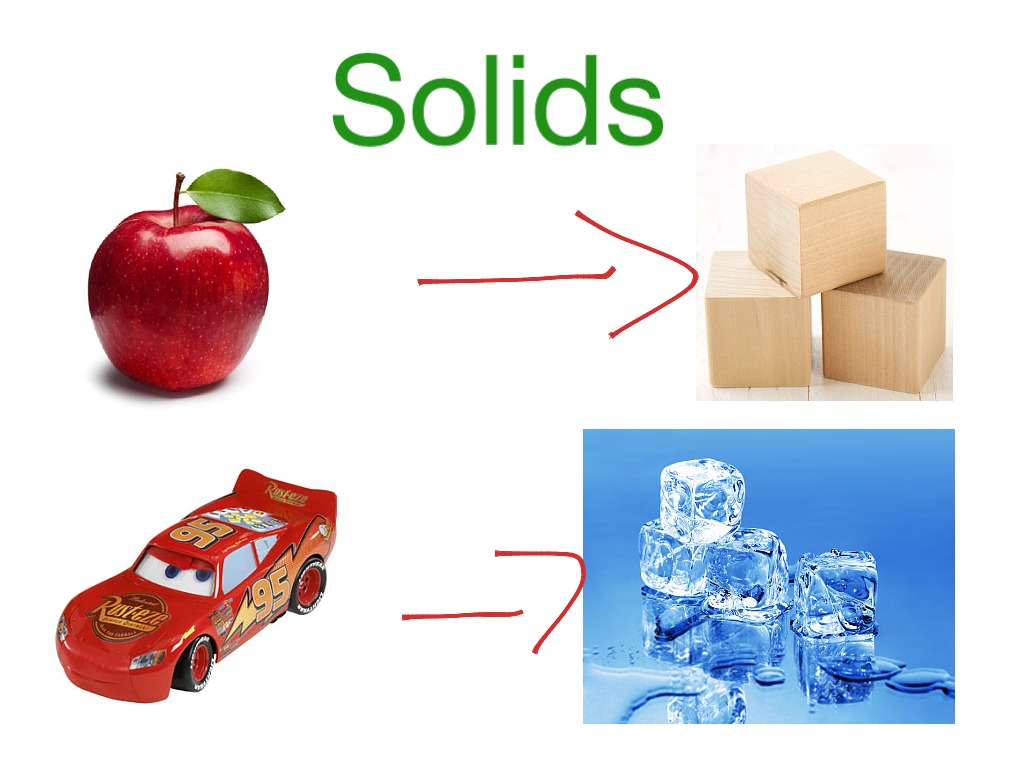
Solids have a definite shape and don’t take the shape of the vessel like liquids or gases.Ī solid can transform into a liquid through melting, and a liquid can transform into a solid through freezing. So, in a solid, the atoms have very low kinetic energy.Īs the atoms are packed in there, solids have high density and it is very difficult to compress solids. Ionic compounds, simple molecules, giant molecules and metals.Solid is a state of matter wherein the atoms and molecules or packed very tightly and the force between particles is so strong that they can’t move, they can just vibrate. The particles in the diagrams could be atoms or molecules or ions, depending on the type of substance, eg In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. The model is used to explain the physical properties of solids, liquids and gases.

The kinetic particle theory of matter is a model that describes the arrangement, movement and energy of particles in a substance.


 0 kommentar(er)
0 kommentar(er)
